Effects of Physical Activity on Reducing Depression and Menopausal Symptoms: A Meta-Analysis
Article information
Abstract
Purpose
This study systematically reviewed the effects of physical activity on depression and various menopausal symptoms in middle-aged women. We aimed to identify physical activity as a useful intervention for mitigating the physiological and psychological challenges associated with menopause and promoting healthy aging.
Methods
Electronic searches were conducted in the MEDLINE, EMBASE, CINAHL, and the Cochrane Library databases using predefined keywords “menopause” and “physical activities”. Of the 1,391 initial articles, 15 randomized controlled trials involving 1,692 middle-aged women were included.
Results
Physical activities led to a reduction in depressive symptoms, with a standardized mean difference (SMD) of -0.60 (95% CI, -0.90 to -0.30). Moreover, all menopausal symptoms, except vasomotor symptoms, were decreased. Specifically, the SMDs for the five subscales of menopausal symptoms were as follows: total scores: -1.53 (95% CI, -2.57 to -0.49); vasomotor: -0.76 (95% CI, -1.53 to 0.00); psychological: -0.93 (95% CI, -1.62 to -0.25); physical: -1.10 (95% CI, -1.77 to -0.43); and urogenital/sexual: -0.67 (95% CI, -1.23 to -0.12).
Conclusion
Physical activity is beneficial for middle-aged women transitioning from peri- to post-menopause. Engagement in physical activity can contribute to the maintenance of overall health and well-being during aging by reducing depression and menopausal symptoms.
INTRODUCTION
As individuals age, they encounter various challenges and adapt to overcome them. While middle age typically results in greater economic stability [1], health-related problems may present major challenges owing to changes in the physical, psychological, and social roles [2]. Women, especially middle-aged women, experience a progression of reproductive aging called menopause [3]. Menopause, a natural stage in the life cycle of women causes both physiological and psychological effects, although it originates in the reproductive system [4].
After the age of 40, structural and functional changes occur gradually in a woman's body. Physiologically, ovarian function gradually declines over a span of ten years, with an abrupt loss of estrogen in the last one year or two years preceding menopause [5]. Menopausal women often experience somatic symptoms, such as hot flushes, headache, sleep disruption, night sweats, sexual problems, cognitive decline, and vaginal dryness, as well as psychological symptoms, including depression, feelings of hopelessness, and anxiety [5,6]. A decline in estrogen levels, which have neuromodulatory effects, may increase the risk of mood disorders in middle-aged women. Reproductive aging is a progressive process, rather than a series of discrete events [7]. Events associated with the menopausal transition, although distinct when observed retrospectively, are indistinct when viewed prospectively [8]. The prevalence of menopausal symptoms among middle-aged women demonstrates interindividual variability [9].
Twenty-two cross-sectional and longitudinal studies examining depression and menopause have indicated a correlation between the menopausal transition and an elevated susceptibility for mood disorders, highlighting the importance of managing mood disorders in peri- and post-menopausal females [8]. Depression and the aggravation of menopausal symptoms may result in decreased physical activity, leading to chronic diseases, such as diabetes, hypertension, osteoporosis, and cardiovascular disease, which can increase mortality [10].
Given that the years spanning middle age constitute more than half of the life cycle [11], the health of individuals during this phase exerts a considerable influence on their health in older age. Therefore, interventions aimed at preserving the physical and mental health of middle-aged individuals are imperative [12,13]. The World Health Organization (WHO) recommends physical activity as a lifestyle change and intervention for middle-aged women [14]. Physical activity resulting in energy expenditure due to musculoskeletal movement is not only effective for middle-aged women, but the benefits may also be greater for these women than for the other age groups [12].
While active media reporting has led to a rise in women's awareness regarding the advantages of physical activity [15], there is insufficient evidence that awareness leads to actual practice [16]. Although physical activity is less common among women than men [17], promoting physical activity available to anyone may alleviate depression or menopausal symptoms that interfere with the daily lives of middle-aged women. Some systematic reviews (SRs) have confirmed the impact of exercise on post-menopausal women, but these studies included post-menopausal women with one or more symptoms of menopause, regardless of age [18,19]. In addition, since these SRs focused only on vasomotor symptoms or measured the overall quality of life, they were not adequate enough to prove the impact of physical activity on menopausal symptoms among women in their middle age in the process of reproductive aging. Although the effects of exercise on depression are known, this study focuses specifically on these effects in middle-aged women.
Therefore, we conducted this review to confirm the evidence regarding the effects of physical activity on depression and menopausal symptoms in randomized controlled trials (RCTs) targeting women aged between 40 to 65 years.
METHODS
This study followed the guidelines of the Cochrane Handbook for Systematic Reviews of Interventions [20] and Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 Statement [21].
1. Search Strategies
A systematic search was conducted in electronic databases, including Ovid-Medline, EMBASE, CINAHL, and Cochrane Library on November 19, 2019 and April 9, 2022. Both searches used the same terms for middle-aged women and physical activity (exercise). MeSH terms were employed in exploded searches, and a variety of text words related to the search terms were explored collectively within the relevant references. The MeSH terms included menopause, premenopause, perimenopause, postmenopause, climacteric, exercise, dancing, muscle stretching, physical exertion, physical fitness, bicycling, running, and walking. We used search techniques including Boolean operators, proximity operators, and truncation for efficient retrieval. The search filters from the Scottish Intercollegiate Guidelines Network [22] were implemented for RCTs in Ovid-Medline. The publication types for RCTs provided by each search engine were utilized across other databases. In the final step, the participants were limited to middle-aged women aged 40~65 years; for all search engines, this criterion for middle age was applicable to all search engines. The search strategy employed in this study is described in Supplementary Table 1.
2. Eligibility Criteria
Literature search was conducted using the following inclusion criteria: (i) Types of studies: RCTs; (ii) Type of participants: middle-aged women ranging in age from 40 to 65 years who had no other medical conditions requiring treatment and had not undergone hormone replacement therapy (HRT) within the past six months, given the potential influence of HRT on depression and menopausal symptoms [23]; (iii) Types of interventions: any physical activity that expends energy through musculoskeletal movement. Physical activity was classified into either aerobic or other activities; (iv) Comparators: waist list control, non-exercise intervention, and usual activity; and (v) Types of outcome measures: depressive symptoms assessed using depression assessment tools, and menopausal symptoms (total, vasomotor, psychological, somatic [physical], and urogenital [sexual] scores) measured using menopause-specific quality of life questionnaires.
The following criteria were used for exclusion from this study: (i) non-original studies, such as letters, editorials, and reviews; (ii) studies that included women younger than 40 or older than 65 years of age; (iii) studies conducted in combination with interventions, such as drug therapy or diet; (iv) studies that compared other types of exercises; and (v) studies involving non-human participants. No limitations were imposed on the utilization of language.
3. Study Selection and Data Extraction
Duplicate articles were excluded, followed by a review of the titles and abstracts. When the title and abstract were not sufficient to make accurate judgements, complete manuscripts were obtained for thorough examination and evaluated based on the inclusion/exclusion criteria. Referring to the Cochrane data extraction forms, an evidence table was prepared for the purpose of extracting data. The extracted data encompassed details, such as the author, publication year, research target, age, location, follow-up period, exercise type, depression, and menopausal symptoms. All procedural steps were independently reviewed by two authors (SHP and YJJ). If the extracted data differed, all authors thoroughly reviewed the full text and achieved a consensus through discussion.
4. Quality Assessment
The assessment of study quality was conducted utilizing the Cochrane Risk of Bias (RoB) 2.0 [24], which is a quality assessment tool designed for RCTs. The five domains encompassed by this instrument are as follows: First, there is the domain of bias arising from the randomization process. Second, we observe bias resulting from deviations in the intended interventions results. Third, we encounter bias in the outcome measurement. Fourth, there is bias resulting from missing outcome data. Lastly, we identify bias in the selection of reported results. Using this instrument, the risk of bias for each signaling question is answered in the form of “yes,” “probably yes,” “probably no,” or “no information.” Following the completion of domain-specific signaling questions, the following three distinct levels of risk-of-bias judgments were determined: high risk of bias, some concerns, and low risk of bias. All steps of the process were independently reviewed by two authors (SHP and YJJ), and any discrepancies were resolved through collaborative discussion among all authors.
5. Statistical Analysis
Cochrane Review Manager (RevMan) version 5.4.1 [25] was used for the meta-analysis. The effect estimates were analyzed utilizing the general inverse variance estimation method, which is based on the random-effects model. Because the tools for each indicator differed, the findings were elucidated using standardized mean differences (SMDs) and 95% confidence intervals (CIs). SMD serves as a summary statistic for standardizing research results with a single scale when the same results are measured with various measurement tools, and it is calculated by dividing the difference in the mean outcome between groups by the standard deviation of outcome among participants [26]. In cases where only the mean and 95% CI were provided, the results were converted into standard deviations using the RevMan calculator tool. The differences between the groups were analyzed using statistical methods with a significance level set at 5%. A small effect size was defined as an SMD less than 0.2. Similarly, an SMD of 0.5 was deemed to represent a medium effect size, while an SMD of 0.8 was categorized as a large effect size.[26]. Heterogeneity among the studies was assessed by utilizing Higgins’ I2 statistics and Cochran’s Q test. The criteria for interpreting I2 were as follows: Firstly, low heterogeneity was defined when the I2 value was equal to or less than 25%. Secondly, moderate heterogeneity was determined when the I2 value fell within the range of 25% to 75%. Lastly, high heterogeneity was identified when the I2 value exceeded 75% or more [27].
RESULTS
1. Selection Process
The search was conducted twice. A total of 2,213 articles were identified, with 1,564 and 649 articles reviewed in the first and second searches, respectively. Two additional articles were identified based on the reference list. After excluding duplicates (n=824), the titles and abstracts of 1,391 articles were subjected to a thorough review, and 124 articles were identified based on a full-text review. Finally, 1,376 articles (99.1%) were excluded, and the total of 15 studies [a1-a15] were included in the analysis. Figure 1 presents a flow diagram illustrating the study inclusion process.
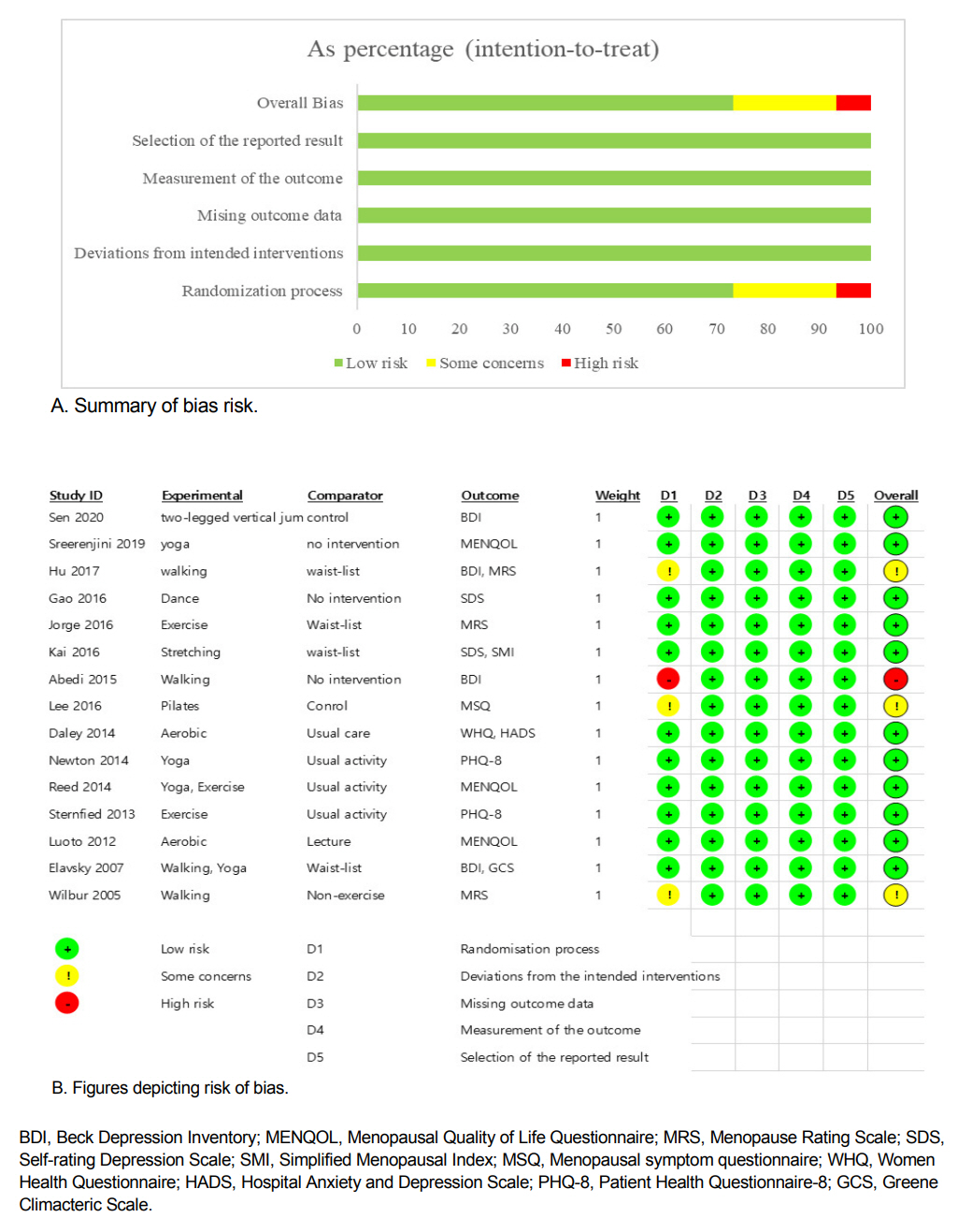
2. Assessing the Risk Of Bias
Quality assessment of the 15 selected studies revealed that 11 studies were labeled as having a low risk of overall bias by satisfying all five domains. Random number tables [a4], lottery methods [a2], and computer assignment methods [a1,a5,a6,a13,a14] were used in the randomization process in these studies. In five studies, blinding was conducted with assessors [a5,a14] or participants [a10-a12]. The remaining four studies were determined to have a risk of bias during the randomization process. The study conducted by Abedi et al. [a8] was determined to exhibit a high risk of bias due to the allocation of odd numbers to the experimental group and even numbers to the control group, based on the health record numbers. The risk of bias was evaluated in three studies [a3,a7,a15] because there was no description of randomization or concealment of the assignment order aside from the author's mention of RCTs. Except for this domain, all other domains were assessed to have a low risk of bias in the three studies (Figure 2).
3. Characteristics of the Selected Studies
The 15 selected studies (encompassing 1,692 middle-aged women) reported the effects of physical activity on depressive and menopausal symptoms, and they were published in the following eight countries: five in the United States; two each in China, Finland, and Japan; and one each in India, Iran, Korea, and the UK. The participants were middle-aged women in the transition phase of menopause who were not receiving hormone therapy, and their average age was 50 years and above. Ten studies had more than 100 participants, and none of the studies had fewer than 30 participants.
The types of physical activities in the selected studies were divided into aerobic activities, such as walking and dancing, and other activities, including yoga, Pilates, and stretching. Three studies [a5,a11,a14] were three-arm RCTs that included both types of activities. Physical activity was performed more than three times per week in most studies. The duration of physical activity varied from 10 minutes in one study [a6], 30~60 minutes in eight studies [a1-a3,a7,a12,a13,a15], and more than 60 minutes in five studies [a5,a10,a11,a12,a14]. One study did not report the duration of physical activity. The follow-up period was mostly 12~16 weeks and 24 weeks, in nine studies and four studies, respectively. Participants in the control groups continued their usual daily activities.
Nine studies reported depressive symptoms. The tools used to measure depression included the Beck Depression Inventory (BDI), Self-Rating Depression Scale (SDS), eight-item Patient Health Questionnaire depression scale (PHQ -8), and Hospital Anxiety and Depression Scale (HADS). Menopausal symptoms were reported in 10 studies; and the tools for measuring these symptoms included the Greene Climacteric Scale (GCS), Menopause Rating Scale (MRS), Menopause-Specific Quality of Life Questionnaire (MENQOL), Menopausal Symptom Questionnaire (MSQ), Simplified Menopausal Index (SMI), and Women Health Questionnaire (WHQ) (Table 1).
4. Effect of Physical Activity on Depressive Symptoms
Nine studies (1,099 women) reported depressive symptoms (Figure 3). As a result of the meta-analysis, SMD was decreased to -0.60 (95% CI: -0.90~-0.30)(Z=3.92, p<.001), and the heterogeneity between the studies (I2) was 82.0% (Tau2=0.18, x2=49.72, p<.001). The sub-group analysis of aerobic activities showed a SMD of -0.74 (95% CI: -1.17~-0.31) (Z=3.40, p=.0007) and I2=86.0%(Tau2=0.28, x2=43.94, p<.001). Other activities showed a SMD of -0.26 (95% CI: -0.48~-0.05) (Z=2.39, p=.02) and I2=6.0% (Tau2=0.00, x2=2.12, p=.35).
5. Effect of Physical Activity on Menopausal Symptoms
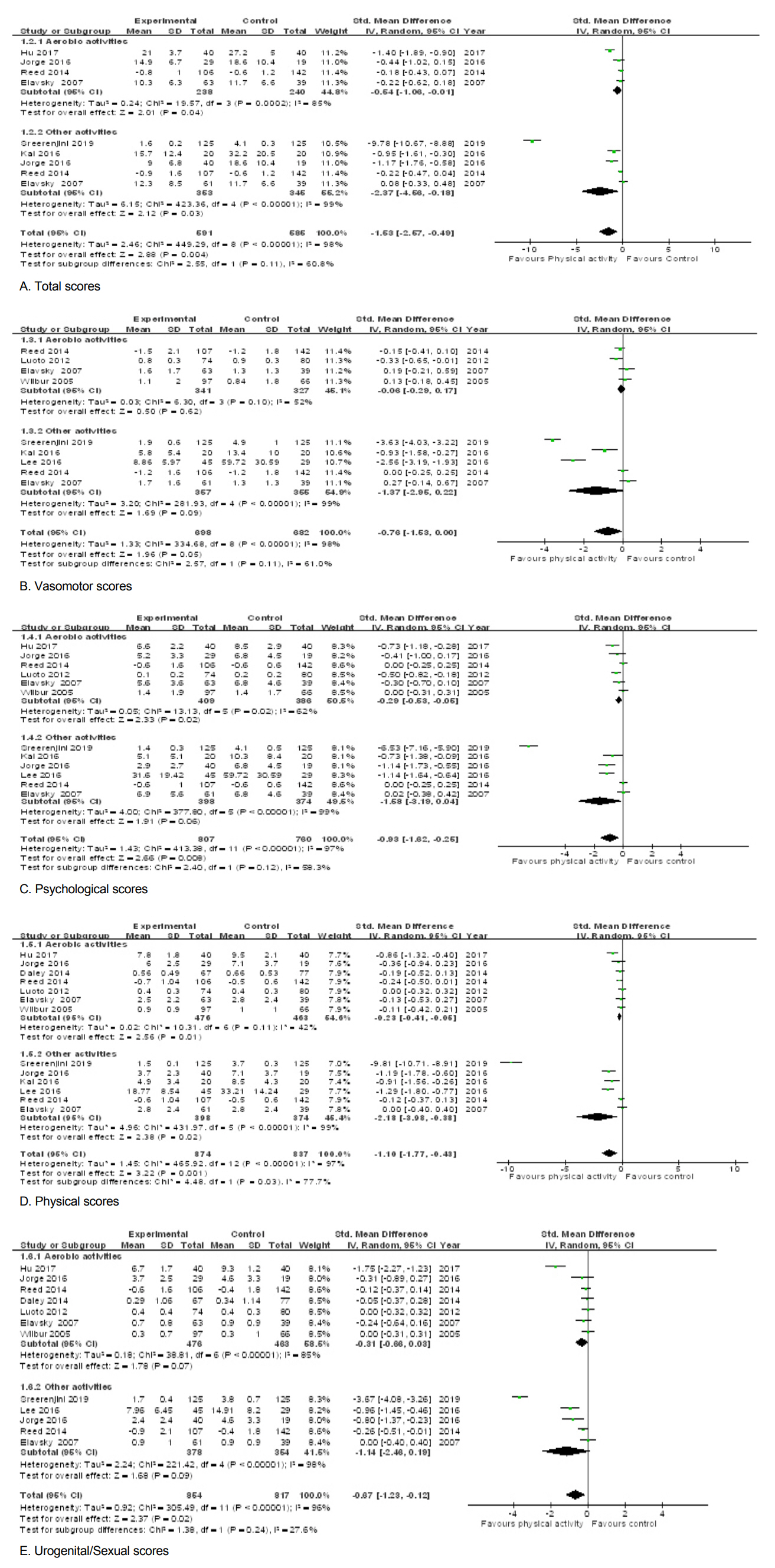
• Total scores. Six studies (976 women) reported the total scores (Figure 4-A). The overall meta-analysis showed a SMD of –1.53 (95% CI: -2.57~-0.49) (Z=2.88, p=.004) and I2=98.0% (Tau2=2.46, x2=449.29, p<.001). The sub-group analysis of aerobic activities showed a SMD of –0.54 (95% CI: -1.06~-0.01) (Z=2.10, p=.04) and I2=85.0% (Tau2=0.24, x2=19.57, p=.0002). Other activities showed a SMD of –2.37 (95% CI: -4.56~-0.18) (Z=2.12, p=.03) and I2=99.0% (Tau2=6.15, x2=423.36, p<.001).
• Vasomotor scores. Seven studies (1,199 women) reported the vasomotor scores (Figure 4-B). The overall meta-analysis showed a SMD of -0.76(95% CI: -1.53~0.00) (Z=1.96, p=.05) and I2=98.0% (Tau2=1.33, x2=334.68, p<.001). The sub-group analysis of aerobic activities showed a SMD of –0.06 (95% CI: -0.29~0.17) (Z=0.50, p=.62) and I2=52.0%(Tau2=0.03, x2=6.30, p=10). Other activities showed a SMD of –1.37 (95% CI: -2.95~0.22) (Z=1.69, p=.09) and I2=99.0% (Tau2=3.20, x2=281.93, p<.001).
• Psychological scores. Nine studies (1,367 women) reported the psychological scores(Figure 4-C). The overall meta-analysis showed a SMD of –0.93 (95% CI: -1.62~-0.25) (Z=2.66, p=.008) and I2=97.0% (Tau2=1.43, x2=413.38, p<.001). In the sub-group analysis of aerobic activities, the SMD was –0.29 (95% CI: -0.53~-0.05) (Z=2.33, p=.02), I2=62.0% (Tau2=0.05, x2=13.13, p=.02). Other activities showed a SMD of –1.58 (95% CI: -3.19~0.04) (Z=1.91, p=.06) and I2=99.0% (Tau2=4.00, x2=377.80, p<.001).
• Physical scores. Ten studies (1,482 women) reported the physical scores (Figure 4-D). The overall meta-analysis showed a SMD of –1.10 (95% CI: -1.77~-0.43) (Z=3.22, p=.001) and I2=98.0%(Tau2=1.45, x2=465.92, p<.001). The sub-group analysis of aerobic activities showed a SMD of –0.23 (95% CI: -0.41~-0.05) (Z=2.56, p=.01) and I2=42.0% (Tau2=0.02, x2=10.31, p=.11). Other activities showed a SMD of –2.18 (95% CI: -3.98~-0.38) (Z=2.38, p=.02) and I2=99.0% (Tau2=4.96, x2=431.97, p<.001).
• Urogenital/Sexual scores. Nine studies (1,471 women) reported the urogenital/sexual scores (Figure 4-E). The overall meta-analysis showed a SMD of –0.67 (95% CI: -1.23~-0.12) (Z=2.37, p=.02) and I2=96.0% (Tau2=0.92, x2=305.49, p<.001). In the sub-group analysis of aerobic activities, SMD was –0.31 (95% CI: -0.66~0.03) (Z=1.78 p=.07) and I2=85.0% (Tau2=0.18, x2=38.81, p<.001). Other activities showed a SMD of –1.14 (95% CI: -2.46~0.19) (Z=1.68, p=.09) and I2=98.0% (Tau2=2.24, x2=221.42, p<.001).
DISCUSSION
Most middle-aged women in the menopausal transition experience depression or menopausal symptoms, such as night sweats or hot flushes [5,6]. While hormone replacement therapy (HRT) has been considered the optimal treatment for alleviating menopausal symptoms, its use is accompanied by potential side effects, and many women seek alternative interventions with fewer risks [19,28]. In this pursuit of scientific evidence for lifestyle-modification approaches to alleviate menopausal and depressive symptoms, this study examined 15 RCTs involving 1,692 middle-aged women. Through a rigorous meta-analysis, our findings revealed that physical activity can effectively reduce depression and menopausal symptoms among middle-aged women aged 40~65 years.
The quality of the selected studies was evaluated using the revised RoB, version 2. Most of the selected studies (11 studies, 73.3%) demonstrated a low risk of bias, and evaluation of the remaining four studies unveiled concerns or a high risk of bias arising from inadequate description of the randomization process. Although blinding was employed to measure the outcomes in only five studies, the tools used to evaluate depression and menopausal symptoms were self-rating scales; therefore, it was concluded that blinding did not affect the outcomes. Thus, the selected studies were well-designed RCTs.
On average, the middle-aged female participants in the 15 selected studies were in their 50s. All participants enrolled in the selected studies had the general characteristics of middle-aged women undergoing menopausal transition, experiencing natural menopause for the last 6~12 months or having estrogen deficiency due to hysterectomy. Participants’ physical activities were categorized as either aerobic or other activity. Aerobic activities were mainly moderate-intensity exercises, such as walking, aerobics, and dancing; some studies included high-intensity training activities, such as jumping rope and jogging, while other studies included yoga, Pilates, and stretching. The frequency and duration of exercise differed among the selected studies. However, the total amount of physical activity per week was approximately 30 min per day. Middle-aged women can easily perform such physical activities, and the WHO [29] recommends a level of physical activity that is consistently accessible.
Physical activity, which middle-aged women can easily perform, reduces both depression and menopausal symptoms. First, depressive symptoms were decreased by 0.60, indicating a medium effect size. Depressive symptoms were assessed using the BDI, SDS, and PHQ-8. These tools are widely used and validated self-rating scales developed to screen for major depressive disorders [30]. Depressive symptoms were decreased in all nine studies (10 cases), but no statistically significant difference was observed across the four studies (five cases). Although there were no common characteristics among the studies, studies that demonstrated statistically significant differences were published relatively recently, after 2015.
Second, menopausal symptoms were reduced in all subscale measures. The tools utilized to assess menopausal symptoms were the Greene Climacteric Scale (GCS), Menopause Rating Scale (MRS), Menopause-Specific Quality of Life Questionnaire (MENQOL), Menopausal Symptom Questionnaire (MSQ), Simplified Menopausal Index (SMI), and Women Health Questionnaire (WHQ). Although the names of the menopausal symptoms in each tool were different, the subscales were similar. For example, physical scores were expressed as somatic (GCS, MRS, SMI, and WHQ) or physical (MENQOL and MSQ) scores, and the measures included fatigue, muscle, and joint pain. Urogenital/sexual scores were referred to as sexual (GCS, MENQOL, and WHQ) or urogenital (MRS and MSQ) scores, and the items measuring sexual symptoms were similar; decreased sexual desire, vaginal dryness, and voiding problems. Only the SMI has no subscale scores corresponding to sexual symptoms. Across all six measurement tools, lower scores indicated fewer menopausal symptoms.
For the subscales, the total score was decreased by -1.53, physical scores by -1.10, and psychological scores by -0.93, all of which were statistically significant with effect sizes greater than 0.8. The effect sizes for the vasomotor and psychological scores were medium at 0.76 and 0.67, respectively. In addition, the overall psychological score was statistically significant; however, the vasomotor score was not statistically significant. In the subgroup-analysis examining the type of physical activity, the effect size of aerobic activity was much smaller than that of the other activities, but the heterogeneity among studies was relatively low. However, the effect sizes of the other activities were greater than 0.8, and the heterogeneity between studies was 98~99%.
Based on this review, practitioners should consider the importance and effectiveness of physical activity in middle-aged women. This study offers insights into the impact of physical activity on various aspects of depression and menopausal symptoms in middle-aged women, and it may guide the development of more targeted strategies for promoting physical activity within this demographic.
However, this study had several limitations. The meta-analysis encompassed a small number of studies, comprising only 15 RCTs. The heterogeneity among selected studies was high (>75%), but subgroup analysis was conducted only for the type of physical activity. Although subgroup analysis could be conducted based on follow-up, the number of studies in each subgroup would be as few as two or three. Therefore, the study’s outcomes and analyses were not sufficient to explain the differences or heterogeneity. Physical activity often has low adherence; however, most studies included in this review performed follow-ups for approximately three months, and only four studies had a six-month follow-up period [a1,a9,a13,a15]. Despite these limitations, this review makes a significant contribution to the literature. Although the effects of physical activity in middle-aged women varied among individual studies, when 15 small-scale short-term studies were integrated and analyzed, the meta-analysis demonstrated that depression and menopausal symptoms experienced by middle-aged female participants could be reduced.
CONCLUSION
Middle-aged women who experience life transitions involving physical, mental, and social changes require effective interventions to maintain or improve their health as they age. This study measured the effectiveness of physical activity in reducing the severity of depression and menopausal symptoms via a systematic review of 15 RCTs. In all of the selected studies, middle-aged women who engaged in physical activity experienced a reduction in depression and menopausal symptom scores. Although menopause is not the only factor threatening the health of middle-aged women, the finding that physical activity three times a week could reduce depression and menopausal symptoms in middle-aged women and have a positive effect on their health is significant. This review may serve as support and motivation for middle-aged women to consistently start and practice physical activity. The results of this study may provide a foundation for women to build and lead healthy later-stage lives.
Notes
Han, KuemSun has served as an editorial board member since March 2021 but played no part in the decision to publish this article. Additionally, no potential conflict of interest relevant to this article was reported
AUTHOR CONTRIBUTIONS
Conceptualization or/and Methodology: Park S-H, Han KS & Jang YJ
Data curation or/and Analysis: Park S-H, Han KS & Jang YJ
Funding acquisition: Park S-H
Investigation: Park S-H & Jang YJ
Project administration or/and Supervision: Park S-H & Han KS
Resources or/and Software: Park S-H & Jang YJ
Validation: Park S-H, Han KS & Jang YJ
Visualization: Park S-H
Writing: original draft or/and review & edition: Park S-H, Han KS & Jang YJ