Mental Health Status among Individuals with Spouses Residing in Long-term Care Facilities: Using Propensity Score Matching
Article information
Abstract
Purpose
The number of older adults with dementia who reside in long-term care institutions has significantly grown. When older adults with dementia are institutionalized, their spouses, who were providing care at home, may experience chronic mental health conditions. This study examined and compared the mental health status of older adults who institutionalized their spouses with dementia in long-term care institutions versus that of older adults who reside with their spouses in their homes.
Methods
The analysis included 95 spouses of institutionalized individuals and 285 control group participants, drawn from 63,617 participants of the 2018 Korean Community Health Survey.
Results
The results showed that the spouses of institutionalized individuals with dementia had more depressive symptoms, poorer sleep quality, and experienced greater stress than those in the control group.
Conclusion
It is necessary to provide supportive care, for alleviating depressive symptoms, poor sleep quality, and stress, to individuals whose spouses are residing in long-term care facilities. For a spouse caregiver, navigation help and education on the changing roles after an older adult’s admission to a long-term care facility would be necessary.
INTRODUCTION
Millions of individuals around the world suffer from dementia [1]. Dementia is an enduring and advancing condition that results in a decline in cognitive abilities beyond the typical effects of natural aging [2]. As the disease progresses, it is accompanied by physical, behavioral, and psychological symptoms and cognitive impairment [3]. Families and partners of those suffering from dementia frequently face challenges dealing with the condition’s symptoms. The effects of dementia, encompassing physical, mental, social, and financial aspects, impact not just individuals with the condition but also their caregivers and families [4].
Adult children generally take care of a parent with dementia. However, the number of spouses caring for partners with dementia is also increasing due to smaller families, lower fertility rates, and rising employment rates [4,5]. Most caregivers are spouses, followed by children and children-in-law [6].
Admittance to a long-term care facility, such as a nursing home, is often prompted by behavioral and psychological symptoms in the individual with dementia as well as distress, depression, and other issues faced by their caregiver [7,8]. About 50% of people with dementia transition from home to long-term care facilities within five years [9]. Despite the objective decrease in the burden of family caregivers following the admission of the older adult with dementia to long-term care facilities, caregivers may experience increased feelings of guilt and depression [10,11]. Even after a person with dementia enters a longterm care facility, their family or spouse’s participation in their care does not completely end. The care of loved ones continues even after admission, although caregivers may experience a change in their role [8,12,13]. Their new role can be to maintain care continuity, which involves helping the older adult maintain their sense of identity through continued love and support from their family ties and helping the institution staff get to know them well. Family members can additionally oversee the situation by observing the care being administered, offering input to the staff, and addressing any care-related deficiencies. They can also engage with the community by interacting with fellow residents, family members, and staff; participating in social gatherings; and essentially establishing a connection with the external environment [13].
Some research results have indicated that caregivers, especially spouses, who institutionalize a family member with dementia have weaker mental health levels than those who continue to care for the individual at home [14]. Spouses may feel relieved due to their decreased responsibilities. Moreover, their emotional distress is lessened due to a reduction in their obligation to provide their spouse with daily life assistance. Despite this, institutionalization may also increase their anxiety, stress, and depression. Additionally, the admission of a spouse to a long-term care facility can cause separation anxiety and adaptation issues among family members, leading to isolation and a weakened support system. Finally, institutionalizing a spouse can increase tension among family members due to the additional economic burden.
Guilt and emotional distress, including anxiety and depression, are further exacerbated when admitting a spouse to a long-term care facility. Though this is expected to reduce caregivers’ emotional and physical burden, recent studies have shown that the adverse effects on emotional or psycho-social outcomes continue even after admission [8,15]. In particular, if perceived stress levels increase, it can affect sleep patterns, cause depression, and ultimately reduce the caregiver’s quality of life [16,17].
Many studies have examined caregivers’ experiences after a spouse with dementia moves into a long-term care facility; however, most studies are qualitative [18-20]. Therefore, this study aimed to confirm the effect of a spouse’s institutionalization on their caregiver’s mental health status through quantitative methods using a nationwide dataset.
This study compared and analyzed the depressive symptoms, sleep quality, and perceived stress levels in older adults who had institutionalized their spouses with dementia in long-term care facilities and typical older adult households. Additionally, the study demonstrates the need for mental health interventions for older adults who remain in the community after their spouses enter long-term care facilities and provides essential data for developing intervention programs.
METHODS
1. Design
This retrospective comparative analysis utilized propensity score matching (PSM) methods and conducted a secondary data analysis of data from the 2018 Korean Community Health Survey (KCHS).
2. Materials and Participants
KCHS refers to the Korea Community Health Survey, an annual national survey carried out by the Korea Disease Control and Prevention agency [21]. Experienced interviewers visit specific households and conduct individual computer-assisted interviews.
In this study, we extracted data on two-person families composed of married couples from the raw data of the 2018 KCHS. The target group was defined as the spouses of individuals with dementia currently residing in longterm care facilities (SwDLF group). The control group comprised individuals who had not been diagnosed with dementia and were living with their spouses in their own homes. The details of the participant selection process are shown in Figure 1.
3. Ethical Considerations
The original data from the KCHS is openly available to the public, and survey participants provided their consent in written form. The data offers anonymous secondary data accessible to the public for academic use.
4. Measurements
1) Depressive symptoms
The Korean version of the Patient Health Questionnaire 9 (PHQ-9) was used to measure depressive symptoms. This questionnaire comprises nine items. The overall score can range from zero to 27, with each item being rated on a scale from zero to three. Higher scores correspond to more severe depressive symptoms. A score of five is considered the threshold for identifying significant depressive symptoms [22].
2) Sleep quality
Sleep quality was evaluated using the Korean version of the Pittsburgh sleep quality index (PSQI-K) [23]. This selfadministered survey serves to quantify sleep quality and disruptions encountered throughout the preceding month. The inventory comprises 19 items, segregated into seven distinct domains, each reflective of the gravity of specific sleep-related challenges [24]. The individual components within seven domains are assessed on a scale ranging from zero to three. Global PSQI score ranges from zero to 21, where elevated scores denote diminished sleep quality. In the PSQI subdomains, sleep latency is presented in minutes, sleep duration in hours, and habitual sleep efficiency in percentage. Additionally, the other subdomains were presented on a scale ranging from zero to three points. In the Korean population, a threshold PSQI global score of six or more indicates poor sleep quality [25]. Cronbach’s ⍺ was .69 in this study.
3) Perceived stress
Perceived stress was measured by the single question: “How much stress do you usually experience in your daily life?”[26]. Participants were instructed to use a 4-point Likert scale ranging from almost never to most of the time. Participants who answered almost never or seldom were categorized as having low stress levels, whereas those who responded with some of the time or most of the time were categorized as having high stress levels.
4) PSM covariates
Ten sociodemographic and health-related characteristics were selected as variables for PSM in the SwDLF and control groups. The PSM covariates included age, gender, education level, monthly household income, residential area, smoking, alcohol intake, obesity, diabetes mellitus, and hypertension. This study divided education level into two categories: those who had not graduated from high school and those who had graduated from high school. Monthly household income was divided into four categories, ranging from Quartile 1 (lowest) to Quartile 4 (highest). Residential area was categorized as either urban or rural. Participants’ obesity status was determined based on their body mass index (BMI). Those with a BMI below 18.5 kg/m2 were categorized as underweight, and those with a BMI of 30 kg/m2 or higher were categorized as obese based on standard cut-off values.
5. Data Analysis
The statistical analyses were performed using SPSS Version 25.0 and R software packages. Categorical variables were presented as frequencies and proportions, while continuous variables were reported as means and standard deviations. The PSM analysis was conducted using the 3:1 nearest neighbor matching method, with the caliper set at 0.1; differences between the two groups were evaluated using the chi-squared test and independent t-test.
RESULTS
1. PSM Analysis and Comparison of Baseline Characteristics
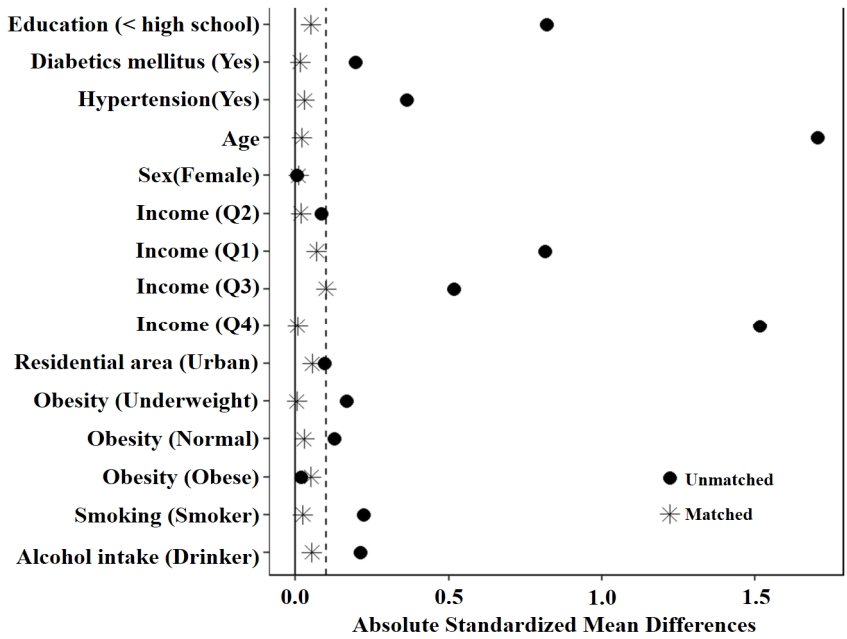
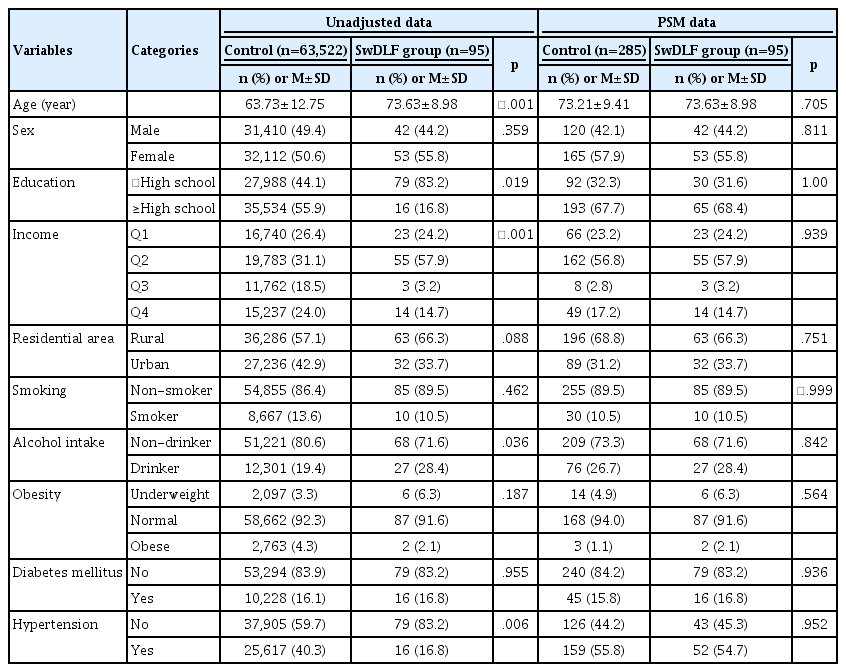
After the PSM analysis, 380 participants were matched, with 95 in the SwDLF group and 285 in the control group. PSM helped eliminate confounding bias that may have been caused by factors related to mental health status and ensured that the SwDLF and control group were comparable. The baseline characteristics of the two groups before and after the PSM analysis are shown in Table 1.
Comparison of Baseline Characteristics Between the Two Groups Before and After Propensity Score Matching (PSM)
Before conducting the PSM analysis, statistically significant differences were observed between the two groups in terms of age (p<.001), education level (p=.019), monthly household income (p<.001), alcohol intake (p=.036), and hypertension (p=.006). However, after the PSM analysis, the baseline characteristics, including age, gender, education level, monthly household income, residential area, smoking, alcohol intake, obesity, diabetes mellitus, and hypertension, did not show any statistically significant differences between the two groups (Figure 2).
2. Comparison of the Mental Health Status of the SwDLF Group and Control Group
Table 2 summarizes the mental health status of the two groups after performing the PSM analysis.
The proportion of individuals with depressive symptoms was significantly higher in the SwDLF group (32.6%, n=31) than in the control group (17.5%, n=50) (x2=4.70, p=.023).
The proportion of participants with poor sleep quality was seen to be significantly higher in the SwDLF group (64.2%, n=64) than in the control group (49.5%, n=141) (x2=6.21, p=.009). The analysis of the PSQI subdomains revealed that the SwDLF group had statistically significantly higher scores for the use of sleep medication (t=-2.05, p=.043) and daytime dysfunction (t=-2.64, p=.009). Although not statistically significant, the SwDLF group had lower sleep latency, duration, and efficiency and higher sleep disturbance scores.
Based on a classification of perceived stress, the proportion of participants who were identified as having high stress level was significantly higher in the SwDLF group (31.6%, n=30) than in the control group (20.7%, n=59) (x2=9.67, p=.004). This finding suggests that a higher proportion of individuals in the SwDLF group experienced high levels of perceived stress.
DISCUSSION
This study evaluated and compared the mental health status of older adults who had institutionalized spouses with dementia in long-term care facilities with those living together in older adult households by analyzing factors such as depressive symptoms, sleep quality, and perceived stress.
First, the proportion of older adults exhibiting depressive symptoms in the SwDLF group was markedly higher than in the control group. Previous studies found that although a caregiver’s burden diminished between six and twelve months following the placement of their spouse in a care facility, their level of depression did not decrease significantly [14]. Institutionalizing patients with dementia in long-term care facilities reduces the objective care burden of spouses. However, depression may arise because spouses must adapt to a new caregiving role. They may also experience difficulty maintaining a continuous relationship with their spouse, managing conflict with the staff in the facility, and dealing with doubts about the quality of services being sustained [27].
Furthermore, guilt over moving a spouse with dementia to a nursing facility and not providing care at home may contribute to depressive symptoms [8,27]. Nevertheless, the importance of providing transitional support to the spouse when older adults with dementia enter longterm care institutions is relatively understated. Therefore, support for spouse caregivers is necessary to help them cope with stress and depressive symptoms and adapt to their new caregiving role during the transition from home to long-term care [28].
Second, this study reveals a notable disparity in sleep quality, with a significantly higher proportion of participants with poor sleep quality observed in the SwDLF group compared to the control group. Previous research has consistently identified diminished sleep quality among spouses or family members providing care to dementia patients within the community [26,29]. However, Von Kanel et al. [30] findings suggest that the death of an individual with dementia substantially reduces total sleep duration for the surviving spouse, accompanied by an increase in nocturnal awakenings. Intriguingly, admission of an older adult to a nursing facility showed no significant relationship to the sleep quality of their spouse. In this study, the PSQI-K global scores exhibited significant differences between the control and SwDLF groups. Furthermore, the elevated prevalence of individuals resorting to sleeping pills and experiencing daytime dysfunction implies a subjective decline in their overall sleep quality.
Third, the study’s findings reveal that the SwDLF group had a markedly higher perceived stress level than the control group. Notably, despite the placement of the older adults with dementia in a long-term care facility in the community, caregiver spouses continued to face novel challenges, such as concerns about the individual with dementia’s adjustment to the nursing facility and participation in family life, which may have contributed to elevated stress levels [18,20]. The spouse's life situation undergoes alterations in tandem with the progression of dementia stages, and some individuals perceive changes in the relationship to be to the extent of that of having undergone divorce or separation. Furthermore, despite the physical presence of the spouse, there is a perceived absence, leading to a feeling of profound loneliness [27]. The stress experienced by spouses tends to escalate with the increasing stages of dementia. According to previous research, from a Clinical Dementia Rating (CDR) of three onwards, the placement of the spouse in a nursing home mitigates caregiving burdens; however, feelings of loss, stress related to self-care, and confusion and despair associated with being alone are heightened [31].
There were several limitations in this study. First, we did not include the characteristics of individuals with dementia. Because this study conducted a secondary data analysis using the 2018 Korean Community Health Survey data, it was unable to incorporate variables related to individuals with dementia that were not included in the original dataset.
Second, we did not consider that the features of older adults living alone may relate to the results for spouses of older adults with dementia. Comparing the mental health status of older adults living alone with that of the spouses of older adults with dementia living in care facilities would help exclude the effects of living alone, as the spouses of older adults with dementia living in long-term care facilities also live alone.
However, this study provides empirical evidence highlighting the necessity of mental health interventions not only for spouses caring for individuals with dementia at home but also for spouses of individuals with dementia residing in long-term care facilities.
CONCLUSION
This study examined the mental health status of the spouses of patients with dementia after the patients’ admission to long-term care facilities. We used the propensity score matching method to reduce selection bias stemming from demographic factors as well as comorbidities that influenced the mental health status of individuals whose spouses had been institutionalized and those who resided with their spouses at home. Our results confirmed that individuals who had primarily provided care at home before the institutionalization of their spouses with dementia continued to be vulnerable in terms of mental health, even after their spouses’ admission to a care facility. This is regarded as a negative consequence of caregiving at home before the individual’s admission to a long-term care facility, as well as the new role that spouses take on after their partner’s admission to such facilities. Therefore, in addition to mental health interventions for community-dwelling individuals with dementia and their spouses, there is a need for mental health promotion and health welfare policies for individuals and families who remain at home after a spouse or family member’s admission a long-term care facility.
Notes
The authors declared no conflicts of interest.
AUTHOR CONTRIBUTIONS
Conceptualization or/and Methodology: Chu, HS
Data curation or/and Analysis: Chu, HS
Funding acquisition: Not applicable
Investigation: Kim, D
Project administration or/and Supervision: Kim, D
Resources or/and Software: Chu, HS
Validation: Kim, D
Visualization: Chu, HS
Writing: original draft or/and review & editing: Chu, HS & Kim, D